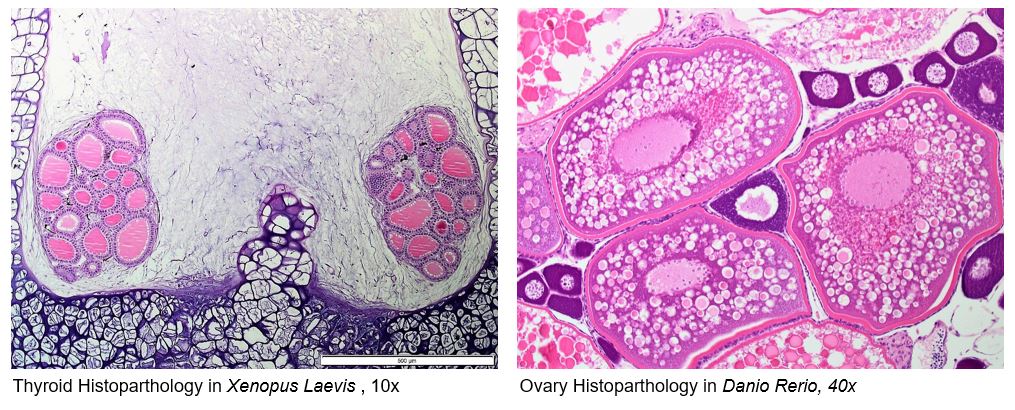
Ecotoxicology – Endocrine Disruptor Histopathology
Endocrine Disrupters (ED) are exogenous substances with a strong effect on the endocrine system (hormone system). It was only in the early 1990 that “Endocrine Disruptor” was coined and since then substantial research was performed leading to the identification of Endocrine Disrupter by validated systems and international regulation was installed.
A 5-level testing strategy is applied to the assessment of EDs by an OECD “Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption”.
Anapath has robust experience in testing ED since AnaPath’s CEO Dr. Klaus Weber has longstanding interest in toxicologic pathology of these compounds, covered by publications starting in 2001.
AnaPath Services will support histopathology in studies like:
- OECD 231 Amphibian Metamorphosis Assay (AMA).
- OECD 241 The Larval Amphibian Growth and Development Assay (LAGDA)
- OECD 229 Fish Short Term Reproduction Assay (FSTRA)
- OECD 234 Fish Sexual Development Test
- OECD 240 Medaka Extended One Generation Reproduction Test (MEOGRT)
- OPPTS 850.1500 Fish Full Lifecyle Test.
Please contact us by sending an email to info@anapath.ch or calling +41 61 906 40 00. We are always available for a conversation and finding the best solution for your project.
AnaPath’s Publications on Endocrine Disruptors